India`s leading research-based health management company with established research, manufacturing and marketing capabilities are collectively known as Panacea Biotec Limited (PBL), which was incorporated on 2nd February 1984 under the name of Panacea Drug (P) Ltd. A second largest vaccine producer in India has the product portfolio of highly innovative prescription products in important therapeutic areas such as pain management, diabetes management, renal-disease management, anti-osteoporosis, anti-tubercular, gastro-intestinal care products and vaccines. The Company has collaborations and tie-ups with leading national and international research organizations and corporations. PBL has ultra modern, state-of-art production facilities at Himachal Pradesh, Punjab & Delhi for manufacturing vaccines and pharmaceutical formulations comply with the US-FDA, UK-MHRA, SAMCC and WHO-cGMP standards and it has four research and development centers. The Company also has 24 product patents, valid in more than 60 countries worldwide.
The plant for vaccines production (Radicura Pharma) and pharmaceutical formulations plant (Panacea Drug P Ltd) at New Delhi were established during the year 1988 and 1989 respectively. In 1993, merger of Panacea Drugs (P) Ltd and Radicura Pharma gave the name Panacea Biotec Ltd. The Company made its Initial Public Offering (IPO) in the year of 1995 and also in the same year, PBL had formed state -of the- art Drug Delivery R&D centre at Lalru. In the year 1997, the company obtained its first product patent in several countries. PBL`s Research & Development made a tie up with European MNC in the year 2001 and in 2002, an in-licensing agreement was made with Biotechnology Consortium of India for development & commercialization of Anthrax Vaccine. Also in the identical year of 2002, the company had commissioned Recombinant Vaccine production plant. During the year 2004, PBL made an in-licensing agreement with National Institute of Immunology, New Delhi, for Japanese Encephalitis Candidate Vaccine, marketing joint venture with Chiron (now Novartis) Vaccines, UK and also made collaboration with Cambridge Bio-stability, UK, for Thermo Stable Vaccines. After a year in 2005, an in-licensing agreement was prepared with National Institute of Health, USA for Hair Growth Hormone.
During the year 2006, ultra modern Pharmaceuticals formulation facility at Baddi, Himachal Pradesh was goes live, which has WHO cGMP complaint. Landmark collaborations were made with The Netherlands Vaccine Institute (The Nederlands Vaccin Institute (NVI)) for manufacture & marketing of finished Inactivated Polio Vaccine (IPV) and a number of IPV based combination vaccines in India and across the globe. Collaboration was happened with PT.Bio Farma to manufacture & market Measles Vaccine. Also in the same year of 2006, the company had inaugurated its Biopharmaceutical R&D Centre at New Delhi and made collaboration with National Research Development Corporation (NRDC) for technology transfer of Foot and Mouth Vaccine. Vaccine Formulation Plant of the company was established at Baddi in the year 2007 and the research agreement was made with Punjab University to develop New Chemical Entities for Psychiatric Disorders. PBL forayed into Healthcare Delivery in the year 2008, made a collaboration to set-up 220 bed multi super-specialty hospital in NCR and also the company obtained a patent (Patent No. 7,371,412 B2) from US Patent & Trademark Office for the product Thank GodTM (Euphorbia Prostrata) for effective management of hemorrhoids & piles in the identical year of 2008.
During year 2008-09, Company`s Joint Venture Company, Chiron Panacea Vaccines Pvt. Ltd. (CPV), set-up for marketing of innovative
combination and other vaccines in India has launched Hepatitis A vaccine HAVpur, in collaboration with Berna Biotech Ltd., Switzerland and the Company`s Injectable Polio Vaccine "PolProtec"and monohib Vaccine (novoHib) in the Indian market.
During the year 2008-09, the Company`s associate firm, viz. M/s Lakshmi & The Manager, in which the Company had invested Rs.40 million (40% share), was taken over by a newly formed company, Lakshmi & Manager Holdings Limited. As a result of takeover of
the said firm, the Company has been allotted Equity Shares for an amount of Rs.41 .3 million in the said company.
The Company had launched generic product Tacrolimus in Germany in 2011 under the brand `Tacpan` through Company`s indirect wholly owned subsidiary company Panacea Biotec Germany GmbH (PBGG).
In 2013, Company`s 50:50 joint venture Adveta Power Pvt. Ltd with its associate PanEra Biotec Pvt. Ltd, incorporated to generate and distribute power or any other energy from conventional / non-conventional energy sources on a commercial basis.
During the year 2013-14, the Company disposed of entire shareholding in its erstwhile WOS, Lakshmi & Manager Holdings Ltd. (LMH). Post such disposal, LMH and its WOS Trinidhi Finance Pvt. Ltd. and subsidiary Best General Insurance Company Ltd. ceased to be the subsidiaries of the Company w.e.f. 25 January, 2014.
During the year 2015, the Company launched new products in various therapeutic categories including: Glizid M V ( Voglibose, Gliclaz ide & Metformin Hydrochloride)- Anti-diabetic; Calcom Plus (Coral Calcium, Vitamin D3 & Vitamin K2-7)- Orthopedic segment and RF Willgo (Paracetamol and Aceclofenac)- Pain management and To Plus (Paracetamol etc.) -Anti-cold.
During 2016, Company introduced India`s first indigenously developed high quality Oncology product, CABAPAN (Cabazitaxel) Injection, for treatment of metastatic Castration Resistant Prostrate Cancer (mCRPC). Further, it introduced indigenously anti-diabetic drug, TENEPAN (Teneligliptin), for treatment of Type 2 Diabetes Mellitus (T2DM). In December 2016, it launched world`s first fully liquid tetravalent vaccine, Easyfour-TT for active primary immunization and booster dose against Diphtheria, Tetanus, Pertussis (DTwP) and Haemophilus Influenza Type B (Hib).
In March, 2017, it launched first liquid whole cell pertussis (wP) based Hexavalent Combination vaccine, EasySixTM. The Company had one subsidiary, viz. NewRise Healthcare Private Limited, which consequently ceased to be subsidiary of the Company effective from April 21, 2017.
The Board of Directors of the Company had, at its meeting held on May 30, 2019 approved the Scheme of Arrangement between Panacea Biotec Limited (Demerged Company) and Ravinder Heights Limited (RVHL) (Resulting Company) and their respective shareholders and creditors for demerger of real estate business of the Company comprising of Radhika Heights Limited along with its step down subsidiaries and two real estate properties, into RVHL, which was approved from the Appointed date, April 01, 2019 and the Scheme became effective from September 10, 2020.
During the current year 2020, Radhika Heights Limited (RHL) along with its step down subsidiaries and two real estate properties, were demerged into RVHL and RHL and its subsidiaries have ceased to be the subsidiary of the Company w.e.f. September 10, 2020.
During the FY 2019-20, the Company transferred its pharmaceutical formulations business including pharmaceutical formulations facility at Baddi and related research & development and natural products extraction activities at Lalru, to its wholly owned subsidiary
viz. Panacea Biotec Pharma Limited (PBPL) by way of slump sale on going concern basis, which completed with effect from February 1, 2020.
As on March 31, 2021, Panacea Biotec Pharma Limited (PBPL ) was reported as the material subsidiary of Company.
The Company and PBPL entered into definitive agreements on February 28, 2022 for sale of Pharmaceutical Formulations Brands
of PBPL in India and Nepal to Mankind Pharma Limited at an aggregate consideration of Rs.18,720 million. As part of this arrangement, Mankind retained PBPL`s well-trained sales and marketing team engaged in the pharmaceutical formulations business and said
transaction was completed on March 3, 2022.
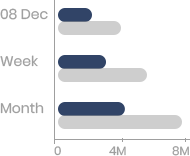
Panacea Biotec share price as on 28 Sep 2024 is Rs. 286.26. Over the past 6 months, the Panacea Biotec share price has increased by 114.75% and in the last one year, it has increased by 78.3%. The 52-week low for Panacea Biotec share price was Rs. 112.35 and 52-week high was Rs. 367.27.
 Invest
Invest